is salt and water homogeneous
The difference between the two is that concentration is the same everywhere in the case of homogeneous mixture whereas in the case of heterogeneous mixture concentration varies from place to. Salt and water solution is a homogeneous mixture.

Eggs Salt Water Water Density Science Experiment Cool Science Experiments Science Experiments Fun Science
Homogeneous mixtures ESAY A solution of salt dissolved in water is an example of a homogeneous mixture.

. Now lets take the example of a saltwater sample. No salt dissolved in water is a homogeneous mixture. Salt water is made by mixing salt in water.
The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. Anything that mixes completely and looks like one layer like salt. Homogeneous mixtures generally termed as solutions have an identical similar occurrence and form throughout the prefix homo means the same.
Answer 1 of 6. Solutions consist of particles as tiny as fragments or molecules. Some other examples of the.
A difference between the two is that concentration is same everywhere in. As weve mentioned earlier saltwater is considered homogeneous because it is uniformly distributed throughout the entire sample. It is a homogeneous mixture and a heterogeneous one.
The amount of salt in the salt water can vary from one sample to another. A difference between the two is that concentration is same everywhere in case of a homogeneous mixture whereas in case of a heterogeneous mixture the concentration varies from place to place within a mixture. Is Saltwater a Homogeneous Mixture.
Hospitals use saline solutions to hydrate patients instead of distilled water because saline solution has sodium as. Is milk heterogeneous or homogeneous. A heterogeneous mixture means you can see the individual components and separate them physically.
Thus salt water is a mixture with salt as the solute and water as the solvent. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. When the salt dissolves it spreads evenly through the water so that all parts of the solution are the same and you can no longer see the salt as being separate from the water.
In fact salt water is a homogeneous mixture and can. If you want to sound more technical is uniform. And salt-water or brine.
A difference between the two is that concentration is same everywhere in case of a homogeneous mixture whereas in case of a heterogeneous mixture the. Answer 1 of 5. It is a homogeneous mixture and a heterogeneous one.
It is a homogeneous mixture and a heterogeneous one. Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. It is because they are both transparent in appearance.
Salt water does not have a uniform composition and we can divide salt and water by simple physical process of evaporation by boiling the salt water. When the salt dissolves it spreads evenly through the water so that all parts of the solution are the same and you can no longer see the salt as being separate from the water. Salt water is made by mixing salt NaCl in water.
The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample. You can see the particles of sand in the water even when you swirl them together. The amount of salt in the salt water can vary from one sample to another.
Homogeneous mixtures ESAY A solution of salt dissolved in water is an example of a homogeneous mixture. Heterogeneous means that you can see specific layers like oil in water. Salt when properly mixed with water doesnt form clusters or regions of different concentration.
We can prepare a salt solution by mixing one or two tablespoons in 1 liter of water. It is a homogeneous mixture and a heterogeneous one. A homogeneous mixture is simply any mixture that is uniform in composition throughout.
In contrast milk would be a homogeneous mixture because you cannot see the individual. A difference between the two is that concentration is same everywhere in case of a homogeneous mixture whereas in case of a heterogeneous mixture the concentration varies from place to place within a mixture. The salt water described above is homogeneous because the dissolved salt is evenly distributed throughout the entire salt water sample.
A salt solution in water is an example of a homogeneous mixture as we cannot see salt particles separately from water. The sea-water contains a mixture of salt and some other bigger size of impurities and a mixture of several gases in seawater. Salt water is made by mixing salt NaCl in water.
Due to other bigger size of impurities seawater is classified as a heterogeneous mixture while due to a mixture of several gases and salt in seawater it is classified as homogeneous mixture. You can get confused between regular water and a saltwater sample. In both cases the solution formed will be homogeneous but the proportions of salt and water are not the same.
Distilled water is created by a process of steaming and condensation and does not contain any minerals like salt calcium or iron. A substance is homogeneous if its composition is identical wherever you sample it - it has uniform composition and properties throughout. Milk for example appears to be homogeneous but when examined under a microscope it clearly consists of tiny globules of fat and protein dispersed in water.
Salt water is made by mixing salt NaCl in water. Often it is easy to confuse a homogeneous mixture with a pure substance because they are both uniform. Salt water is made by mixing salt NaCl in waterIt is a homogeneous mixture and a heterogeneous one.
Why do hospitals use saline instead of distilled water.

Mixtures Homogeneous Heterogeneous And Solutions Power Point Types Of Mixtures Heterogeneous Mixture Chemical And Physical Changes

Heterogeneous And Homogeneous Mixtures Physical Science Matter Winter Holiday Christmas Christmas Science Middle School Science Activities Winter Holidays

Pure Substances And Mixtures Pure Products Elements Compounds And Mixtures Compounds And Mixtures

Physical Science Lessons 5th Grade Science Middle School Science Experiments

Let S Do An Experiment Homogeneous And Heterogeneous Mixtures Heterogeneous Mixture Solutions And Mixtures Physical Changes Experiments

What Is The Meaning Divine Ka Gifts Of The Spirit Knowledge And Wisdom Human Soul

Classification Of Matter Ultimate Card Sort For Pure Substances And Mixtures Sorting Cards Creative Teaching Teacher Preparation

Mel Science On Instagram Mix Some Isopropyl Alcohol And Water In A Bottle To Create A Homogeneous Liquid Add Salt And Food Col Alcohol Science Food Coloring

Solute Solvent Solution For Kids Kindergarten Toddlers Preschoolers Teaching Chemistry Solutions Toddler Preschool
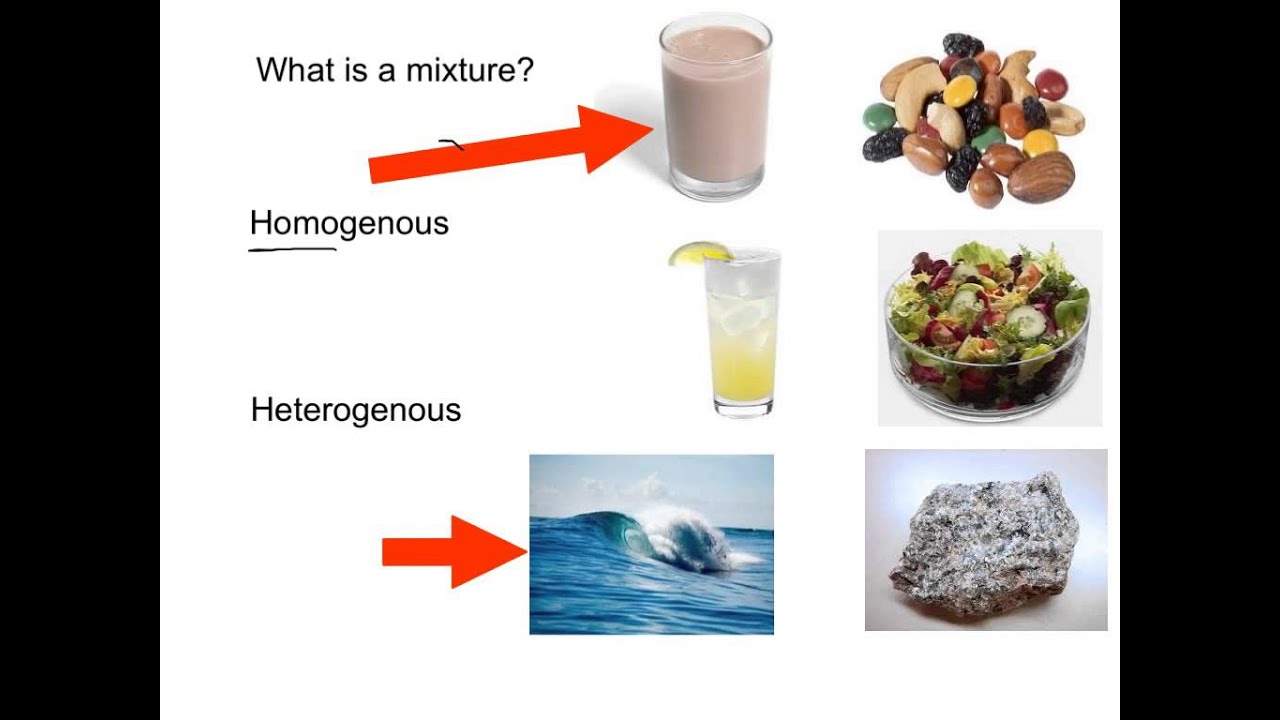
Pin On Middle School Chemistry

Selina Concise Chemistry Class 6 Icse Solutions Chapter 5 Pure Substances And Mixtures Separation Of Mixtures Ncert Chemistry Class Chemistry Science Notes

Homogeneous And Heterogeneous Mixtures Examples Classification Of Matte In 2021 Heterogeneous Mixture Chemistry Organic Chemistry Tutor

Let S Mention The Difference Between A Solution And A Heterogeneous Mixture A Solution Is A Comb Chemical Science Solutions And Mixtures Heterogeneous Mixture

Mixtures And Pure Substances Heterogeneous Mixture Pure Products Mixtures

Is Matter Around Us Pure School Help By Gunjan Pure Products School Help Homogeneous Mixture

Homogeneous And Heterogeneous Mixtures Card Sorting Activity Heterogeneous Mixture Sorting Activities Middle School Science Resources

Pin Di Carrie Flanagan Su School Stuff Tavola Periodica

Grade 5 Science Lesson 8 Matter Mixture And Solution Primary Science Science Lessons Science Primary Science

0 Response to "is salt and water homogeneous"
Post a Comment